What is Zepbound?
Zepbound is the brand name for tirzepatide, an injectable medication administered once weekly. It was first approved by the U.S. FDA in May 2022 under the name Mounjaro for type 2 diabetes. In November 2023, the FDA expanded approval under the name Zepbound for chronic weight management in adults with obesity (BMI ≥30) or overweight (BMI ≥27) with at least one weight-related condition.
Unlike earlier drugs in its class (such as semaglutide in Ozempic or Wegovy), Zepbound uniquely acts on two incretin hormones: GLP-1 and GIP. This dual mechanism produces greater metabolic improvements and more pronounced weight loss than GLP-1 agonists alone.
How Does Zepbound Work?
The Role of GLP-1
GLP-1 is a gut hormone released in response to food intake. It increases insulin secretion, slows gastric emptying, and signals satiety to the brain. This leads to lower blood glucose and reduced appetite.
The Role of GIP
GIP is another hormone that enhances insulin secretion and may improve fat metabolism. When combined with GLP-1 activity, it amplifies the weight loss effect and improves metabolic efficiency.
Dual Action Advantage
By mimicking both hormones, tirzepatide exerts a stronger effect than semaglutide. In trials, patients taking tirzepatide lost significantly more weight than those on placebo or semaglutide, highlighting the added benefit of GIP activation.
Key Benefits of Zepbound
For Diabetes Management
- Significant reductions in A1C (average blood sugar)
- Improved insulin sensitivity
- Lower fasting and post-meal glucose levels
For Weight Loss
- 15%–22% average body weight reduction depending on dose
- Reduced appetite and cravings
- Improved mobility, energy, and quality of life
For Heart and Metabolic Health
- Lower blood pressure and cholesterol
- Improved sleep in patients with sleep apnea
- Potential reduction in long-term cardiovascular risk
Clinical Trial Evidence
SURPASS Trials (Type 2 Diabetes)
The SURPASS program evaluated tirzepatide for type 2 diabetes. Results showed:
| Trial | Patient Group | A1C Reduction | Weight Loss |
|---|---|---|---|
| SURPASS-1 | Type 2 diabetes, no prior treatment | -1.9% to -2.1% | 7–9 kg |
| SURPASS-2 | Compared tirzepatide vs semaglutide | -2.0% vs -1.8% | 12 kg vs 6 kg |
| SURPASS-3/4 | Compared vs insulin | -2.4% vs -1.4% | 12–14 kg vs weight gain |
SURMOUNT Trials (Obesity & Weight Loss)
The SURMOUNT program evaluated tirzepatide in patients with obesity but without diabetes.
| Dose | Average Weight Loss (%) | Percent of Patients Losing ≥20% |
|---|---|---|
| 5 mg | ~15% | 30% |
| 10 mg | ~19% | 45% |
| 15 mg | ~21% | 55% |
| Placebo | ~3% | 3% |
Chart: Average Weight Loss Over 72 Weeks
(Below is a simple HTML representation of trial outcomes. A designer could render this into a branded visual chart.)
| Week | Placebo | 5 mg | 10 mg | 15 mg |
|---|---|---|---|---|
| 24 | -2% | -8% | -10% | -12% |
| 48 | -2.5% | -12% | -16% | -18% |
| 72 | -3% | -15% | -19% | -21% |
Zepbound Dosage and Administration
Zepbound is given once weekly as a subcutaneous injection. It comes in a prefilled pen that patients can use at home.
Injection sites include the abdomen, thigh, or upper arm. Rotating sites helps reduce irritation.
The dosing schedule gradually increases:
| Stage | Dose | Duration |
|---|---|---|
| Starter | 2.5 mg | 4 weeks |
| Titration | 5–10 mg | Every 4 weeks |
| Maintenance | 10–15 mg | Ongoing |
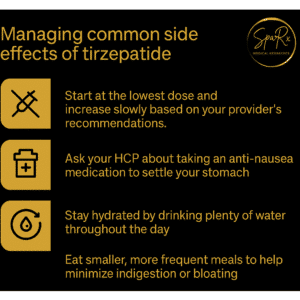
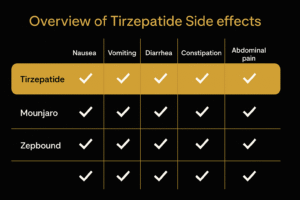
Possible Side Effects of Zepbound
Common Side Effects
- Nausea, vomiting, diarrhea, or constipation
- Loss of appetite
- Fatigue
- Indigestion or bloating
Serious but Rare Risks
- Pancreatitis
- Gallbladder problems
- Kidney injury (from dehydration)
- Thyroid tumors (seen in rodents, human risk unclear)
Most side effects are mild and improve over time. Patients can reduce nausea by eating smaller meals, avoiding greasy foods, and staying hydrated.
 Cost and Insurance Coverage
Cost and Insurance Coverage
Without insurance, Zepbound can cost over $1,000 per month. Coverage is often better for type 2 diabetes than for obesity. Prior authorization is common.
At SpaRx Medical Aesthetics, we assist patients with:
- Manufacturer savings cards
- Pharmacy discount programs
- Exploring compounded alternatives when appropriate
Who Should Not Take Zepbound?
Contraindications include:
- Personal or family history of medullary thyroid carcinoma or MEN2
- Past serious hypersensitivity to tirzepatide
- Pregnancy or breastfeeding
Caution is needed with insulin or sulfonylureas due to risk of hypoglycemia.
Frequently Asked Questions
How quickly does Zepbound work?
Appetite suppression often begins within 2–4 weeks, with weight loss visible by 3 months. Clinical trials demonstrated peak weight loss around 72 weeks.
Can I stop once I reach my goal weight?
Stopping therapy usually results in partial weight regain. Ongoing treatment and lifestyle adjustments are recommended.
How does Zepbound compare to Wegovy or Ozempic?
Zepbound tends to produce greater weight loss than semaglutide due to its dual GIP/GLP-1 mechanism.
Is Zepbound safe for long-term use?
Long-term studies suggest sustained benefits, but ongoing monitoring is important for side effects and metabolic changes.