Tirzepatide Injection (Mounjaro / Zepbound)
Dual-Action Metabolic Therapy for Diabetes & Weight Loss
Tirzepatide is a breakthrough dual-action medication approved for type 2 diabetes and obesity. It improves blood sugar and promotes weight loss by activating GLP-1 and GIP pathways. These mechanisms optimize glycemic control and satiety.

How Tirzepatide Works
Tirzepatide is a dual-action medication mimicking two natural hormones – GLP-1 (Glucagon-Like Peptide-1) and GIP (Glucose-dependent Insulinotropic Polypeptide) – to regulate blood sugar, appetite, and digestion simultaneously.
GLP-1 Pathway
- Enhances insulin secretion post-meal
- Suppresses glucagon to limit liver glucose output
- Slows gastric emptying, increasing satiety
- Reduces appetite via central nervous system
GIP Pathway
- Boosts insulin response only when needed
- Enhances postprandial glycemic control
- Supports satiety signaling in the brain
- Synergistic effect with GLP-1 for dual-action benefits
Side Effects & Safety Considerations
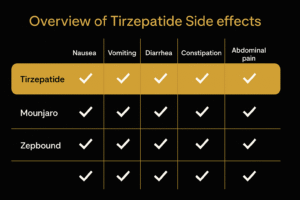
Tirzepatide, like all medications, carries potential side effects. Most are mild to moderate, primarily gastrointestinal, and often improve with continued use or stepwise dose escalation.
- Nausea: Common initially, often resolves over time; mitigated by slow titration and small meals.
- Vomiting & Diarrhea: Usually mild; hydration and dietary adjustments help.
- Constipation: May occur as digestion slows; fiber and fluids help manage.
- Decreased appetite: Part of the therapeutic effect, promoting weight loss.
- Less common risks: Hypoglycemia with concomitant medications, pancreatitis, gallbladder issues, thyroid tumor risk (rodent studies; rare in humans).
- Injection site reactions: Mild redness, itching, or transient discomfort.
Dosing & Injection Tips
- Start at 2.5 mg once weekly; increase every 4 weeks as tolerated up to 15 mg.
- Store pens refrigerated (36–46°F); room temp max 21 days if needed.
- Rotate injection sites: abdomen, thigh, upper arm.
- Auto-injector: remove cap, press pen flat, twist unlock, push plunger until second click (~5–10 sec).
- If a dose is missed: inject within 4 days if possible; otherwise skip and resume next scheduled dose. Do not double doses.
- Check liquid clarity; do not use cloudy or discolored pens.
- Dispose of used pens in sharps container; do not reuse.
Frequently Asked Questions
How quickly will I lose weight on Tirzepatide?
Weight loss is gradual. Patients often see 1–3 lbs per week initially, with the majority of changes occurring by 6–9 months, and peak results by 12–18 months. Individual results vary based on lifestyle, dose, and metabolism.
Can I use Tirzepatide if I don’t have diabetes?
Yes, FDA-approved for chronic weight management in adults with obesity or overweight with comorbidities. BMI ≥30, or ≥27 with weight-related health conditions. Prescription and supervision by a healthcare provider are required.
Do I need a special diet?
No strict diet required, but healthy eating enhances results. Focus on smaller portions, nutrient-dense foods, hydration, and mindful eating. Mediterranean or balanced diets work well.
What happens if I stop taking it?
Effects gradually diminish. Blood sugar may rise, and weight regain is possible. Continuation of healthy habits is important; discuss with your provider if considering discontinuation.
Long-Term Safety & Monitoring
Tirzepatide has been studied up to two years in extension trials with continued benefit and manageable side effects. Regular monitoring of A1C, weight, gastrointestinal tolerance, and labs is recommended. Healthcare providers assess ongoing therapy suitability and adjust as necessary.
Emerging data suggest potential cardiovascular benefits, but ongoing studies are evaluating long-term outcomes for heart disease risk, stroke, and diabetes prevention. Safety remains high when following prescribed dosing and monitoring guidelines.
Final Thoughts
Tirzepatide represents a milestone in diabetes and obesity management, providing dual-action metabolic benefits, clinically significant weight loss, and improved glycemic control. Best results are achieved alongside healthy lifestyle practices. Stepwise dosing, proper storage, and adherence to injection instructions enhance tolerability and efficacy.
This infographic summarizes the complete profile: mechanism, benefits, dosing, safety, and FAQs. Patients and healthcare providers can use it as a reference to understand Tirzepatide’s full clinical potential.
Regulatory Approvals
Tirzepatide has been rigorously evaluated and approved for multiple indications by the FDA:
- Type 2 Diabetes (Mounjaro): Approved in 2022 as an adjunct to diet and exercise for adults with inadequate glycemic control on oral medications.
- Chronic Weight Management (Zepbound): Approved in 2023 for adults with obesity (BMI ≥30) or overweight (BMI ≥27) with at least one weight-related comorbidity such as hypertension, dyslipidemia, or sleep apnea.
These approvals are based on large clinical trials demonstrating meaningful improvements in glycemic control and substantial weight reduction.
Clinical Benefits
The benefits of tirzepatide extend beyond glucose lowering and weight loss. Here is a detailed look at its effects:
- Blood Sugar Control: Average A1C reduction of 2–2.5%, with many patients achieving levels below 7%, reducing the risk of microvascular and macrovascular complications.
- Weight Loss: In SURPASS and SURMOUNT trials, patients achieved 12–25 lbs reduction in diabetes studies, and up to 20% body weight reduction in obesity trials.
- Cardiometabolic Health: Improvements in blood pressure, LDL-C, triglycerides, and waist circumference have been observed alongside weight loss.
- Quality of Life: Patients often report increased energy, better mobility, and enhanced control over eating behavior.
- Diabetes Prevention: In high-risk populations, tirzepatide has been shown to reduce progression from prediabetes to diabetes, likely due to combined glycemic and weight control effects.
- Comparison to Existing Treatments: Compared to semaglutide 1 mg, tirzepatide demonstrated superior A1C reductions and approximately double the weight loss in head-to-head trials.
| Drug | Weight Loss | A1C Drop | Dosing | FDA Approved |
|---|---|---|---|---|
| Tirzepatide | ~22.5% | Up to 2.4% | Weekly | Yes |
| Semaglutide | ~15% | ~1.8% | Weekly | Yes |
Side Effects
While tirzepatide is generally well-tolerated, patients may experience some adverse effects:
- Common: nausea, vomiting, diarrhea, constipation (usually mild to moderate)
- Expected effects due to mechanism: reduced appetite, slowed gastric emptying
- Less common/serious: hypoglycemia (with concomitant medications), pancreatitis, gallbladder issues, thyroid C-cell tumor risk in animals
- Mild/rare: injection site reactions, headache, fatigue
| Side Effect | Tirzepatide | Semaglutide |
|---|---|---|
| Nausea | Yes | Yes |
| Constipation | Some | Moderate |
Dosing Schedule & Injection Guidance
Tirzepatide is administered via a once-weekly subcutaneous injection. Proper dosing and technique are critical for efficacy and minimizing side effects.
Standard Dose Escalation
- Start: 2.5 mg once weekly for 4 weeks to allow body adjustment
- Step-up: 5 mg weekly for next 4 weeks
- Mid-range: 7.5–10 mg weekly depending on tolerability
- Maximum: 15 mg weekly as clinically indicated
| Drug | Starting Dose | Max Dose |
|---|---|---|
| Tirzepatide | 2.5 mg | 15 mg |
| Semaglutide | 0.25 mg | 2.4 mg |
Missed Doses
- If remembered within 4 days (96 hours), take the missed dose immediately
- If >4 days have passed, skip missed dose and resume normal schedule
- Never double-dose to compensate; follow healthcare provider guidance
Storage Instructions
- Refrigerate pens at 36–46°F (2–8°C); keep in original box
- Do not freeze; discard if frozen
- Room temperature (up to 86°F) allowed for max 21 days
- Check expiration date and liquid clarity before use
Injection Process
- Choose site: abdomen, thigh, or upper outer arm
- Clean with alcohol swab and allow to dry
- Remove pen cap, press against skin, twist to unlock, and press plunger
- Hold pen for 5–10 seconds until full dose delivered (listen for second click)
- Rotate injection sites each week to reduce irritation or scar tissue
- Dispose pens in a sharps container; do not reuse
Time to See Results
- Tirzepatide: 4–8 weeks for measurable effects
- Semaglutide: 6–10 weeks
Frequently Asked Questions
1. How quickly will I lose weight on Tirzepatide?
Weight loss is gradual and steady. Most patients notice initial changes within 1–2 months, with progressive reductions over 6–18 months. Typical weekly loss is 1–3 pounds, though individual results vary depending on diet, activity, genetics, and starting weight. Consistent adherence to injection schedule and lifestyle interventions enhances results.
2. Can Tirzepatide be used without diabetes?
Yes — the FDA has approved Tirzepatide (Zepbound) for adults with obesity (BMI ≥30) or overweight (BMI ≥27) with weight-related comorbidities. Weight-loss therapy is guided by clinical evaluation, and only patients meeting criteria are prescribed this medication. It is prescription-only and should be used under medical supervision.
3. Do I need to follow a special diet?
No specific “Tirzepatide diet” is required, but following a nutritious, balanced diet will maximize benefits. Patients often feel less hungry and consume smaller portions naturally. Recommended practices include:
- Eat slowly and mindfully to accommodate slower digestion
- Avoid very greasy or rich foods initially
- Stay hydrated; water intake is essential, especially if vomiting or diarrhea occurs
- Moderate simple carbohydrates and sugars; focus on whole grains and fiber
- Follow signals of fullness to prevent overeating
4. What happens if I stop taking Tirzepatide?
Discontinuing Tirzepatide gradually reduces its effects. Blood sugar may rise and weight can be regained over time. Studies show weight regain is gradual, highlighting obesity as a chronic condition requiring long-term management. Patients should have a structured plan in place and follow provider guidance when stopping medication.
5. Insurance and Cost Considerations
Medication costs vary depending on insurance coverage. Approximate retail pricing:
| Drug | Monthly Cost (USD) | Insurance Coverage |
|---|---|---|
| Tirzepatide | ~$1,100 | Varies by plan |
| Semaglutide | ~$1,300 | Some plans |
6. Is long-term use safe?
Long-term safety data is currently limited to trial durations of 1–2 years. Evidence so far indicates continued efficacy and manageable side effects. Periodic monitoring of A1C, weight, kidney function, and gastrointestinal health is recommended. Ongoing research is evaluating cardiovascular and metabolic benefits over extended periods.
7. Practical Safety Tips
- Follow provider instructions for injections and dose escalation
- Track doses via calendar or app; do not share pens
- Keep pens out of reach of children and pets
- Report any unusual symptoms (abdominal pain, severe nausea, or neck lumps) immediately
- Discuss all medications with your healthcare provider to avoid interactions
- Limit alcohol to moderate intake
Comparison to Other Treatments
Tirzepatide has been directly compared to semaglutide (Ozempic) and other common diabetes medications in clinical trials:
| Drug | Weight Loss | A1C Drop | Dosing | FDA Approved | Notes |
|---|---|---|---|---|---|
| Tirzepatide | ~22.5% | Up to 2.4% | Weekly | Yes | Dual GLP-1 + GIP action; superior weight and A1C reduction |
| Semaglutide | ~15% | ~1.8% | Weekly | Yes | GLP-1 only; effective but less weight loss |
| Daily Insulin | Variable | 1–2% | Daily | Yes | Controls blood sugar but can cause weight gain |
Extended Practical Guidance
- Track progress: Monitor weight, blood sugar, and side effects weekly
- Maintain lifestyle support: Balanced diet and regular physical activity enhance results
- Hydration is key: Especially with gastrointestinal side effects
- Rotate injection sites: Avoid bruising or scar tissue accumulation
- Follow-up appointments: Ensure dosing and side effects are managed by your healthcare provider
- Stay informed: Discuss new research, cardiovascular outcomes, and potential long-term benefits with your provider
Final Thoughts
Tirzepatide (Mounjaro / Zepbound) represents a transformative option for adults managing type 2 diabetes and obesity. Its dual GLP-1 and GIP mechanism provides superior glycemic control and weight reduction compared to current treatments. When combined with a structured diet, exercise, and regular medical follow-up, it can substantially improve metabolic health and quality of life.
While side effects exist, they are generally manageable, and long-term monitoring ensures safety and efficacy. Patients are encouraged to work closely with their healthcare providers to optimize results, manage side effects, and maintain healthy lifestyle habits. Tirzepatide is a powerful tool in chronic disease management — but the partnership between patient, provider, and lifestyle choices remains essential for sustained health benefits.
By integrating Tirzepatide into a comprehensive care plan, patients can achieve meaningful improvements in blood sugar, body weight, and overall cardiometabolic health. This infographic serves as a detailed guide for understanding its mechanisms, benefits, risks, and practical considerations, empowering informed decision-making.